999LUCKY เว็บแทงหวยออนไลน์ อันดับ 1

ในขณะที่เทคโนโลยีก้าวหน้าไปมาก และการใช้ชีวิตในประจำวันเรายังได้มีเทคโนโลยีมามีส่วนร่วยเกือบทุกอย่าง ไม่เว้นแม้แต่การแทงหวยก็ยังสามารถแทงผ่านระบบออนไลน์ได้อีกด้วย โดยที่ท่านไม่จำเป็นต้องออกไปซื้อหวยนอกบ้านให้เสี่ยงอีกต่อไป ท่านเพียงมีคอมพิวเตอร์หรือมือถือสมาร์โฟน ก็สามารถแทงหวยได้ทุกที่ทุกเวลาตามที่ท่านต้องการได้แล้ว และการแทงหวยออนไลน์ ยังมีข้อดีอีกหนึ่งอย่างคือ อัตราการจ่ายเงินรางวัลที่สูง ส่วนลดเปอร์เซ็นต์เยอะ และบางเว็บก็ไม่มีเลขอั้น
เว็บแทงหวยออนไลน์
และเว็บที่เราอยากจะแนะนำให้ทุกท่าน นั่นก็คือ 999LUCKY เว็บแทงหวยออนไลน์ ที่มีความน่าเชื่อถือมากที่สุดในประเทศไทย จ่ายจริง จ่ายตรง การเงินมั่นคง สมัครเลย ฟรี!! ไม่เสียค่าใช้จ่ายใดๆทั้งสิ้น ระบบของเว็บมีความทันสมัย สามารถรองรับการเข้าใช้งานผ่านมือถือสมาร์ทโฟนทุกระบบ หากมีข้อสงสัยหรือต้องการสอบถามข้อมูลท่านสามารถ ติดต่อพนักงาน เว็บหวยออนไลน์อันดับ1 ได้ตลอด 24 ช.ม.
หวยบาทละเท่าไหร่
หวยบาทละเท่าไร คำถามนี้มีคำตอบที่ไม่ตายตัวครับ เพราะแต่ละเว็บ แต่ละเจ้ามือ นั่นมีอัตราการจ่ายที่ไม่เท่ากัน อย่างเจ้ามือที่ซื้อกันตามบ้าน อัตราการจ่ายก็อยู่ที่เลข 3 ตัวตรง บาทละ 450-500 เลข 3 ตัวโต๊ด บาทละ 90-100 เลข 2 ตัว บาทละ 60-65 ไม่เกินนี้ และไม่มีส่วนลด ส่วนอัตราการจ่ายของเว็บจะอยู่ที่ เลข 3 ตัวตรง บาทละ 600-800 เลข 3 โต๊ด บาทละ 100-130 เลข 2 ตัว บาทละ 65-90 และยังสามารถเลือกได้ว่าจะรับอัตราจ่ายเงินรางวัลสูง หรือรับส่วนลดเยอะก็ได้ จะเห็นได้ชัดว่าอัตราการจ่ายเงินรางวัลของเว็บจะจ่ายเยอะกว่ามาก
อัตราจ่ายเงิน 999LUCKY
อัตราการจ่ายเงินรางวัลของเว็บแต่ละเว็บนั้น มีความแต่ต่างกันออกไป อย่าง เว็บแทงหวย999lucky ถือได้ว่าเป็นเว็บที่มีอัตราการจ่ายที่สูงกว่าทุกเว็บแทงหวยในเมืองไทยเลยก็ว่าได้ เพราะมีอัตราจ่ายเลข 3 ตัวตรง อยู่ที่ บาทละ800 เลยทีเดียว และยังมีสเต็ปการแทงให้ท่านสามารถเลือกเล่นได้มากถึง 4 สเต็ป ไม่ว่าจะเลือกรับอัตราการจ่ายสูงสุด หรือเลือกรับส่วนลดมากสุดถึง 40% ท่านก็สามารถเลือกได้ตามแผนการเงินของคุณ
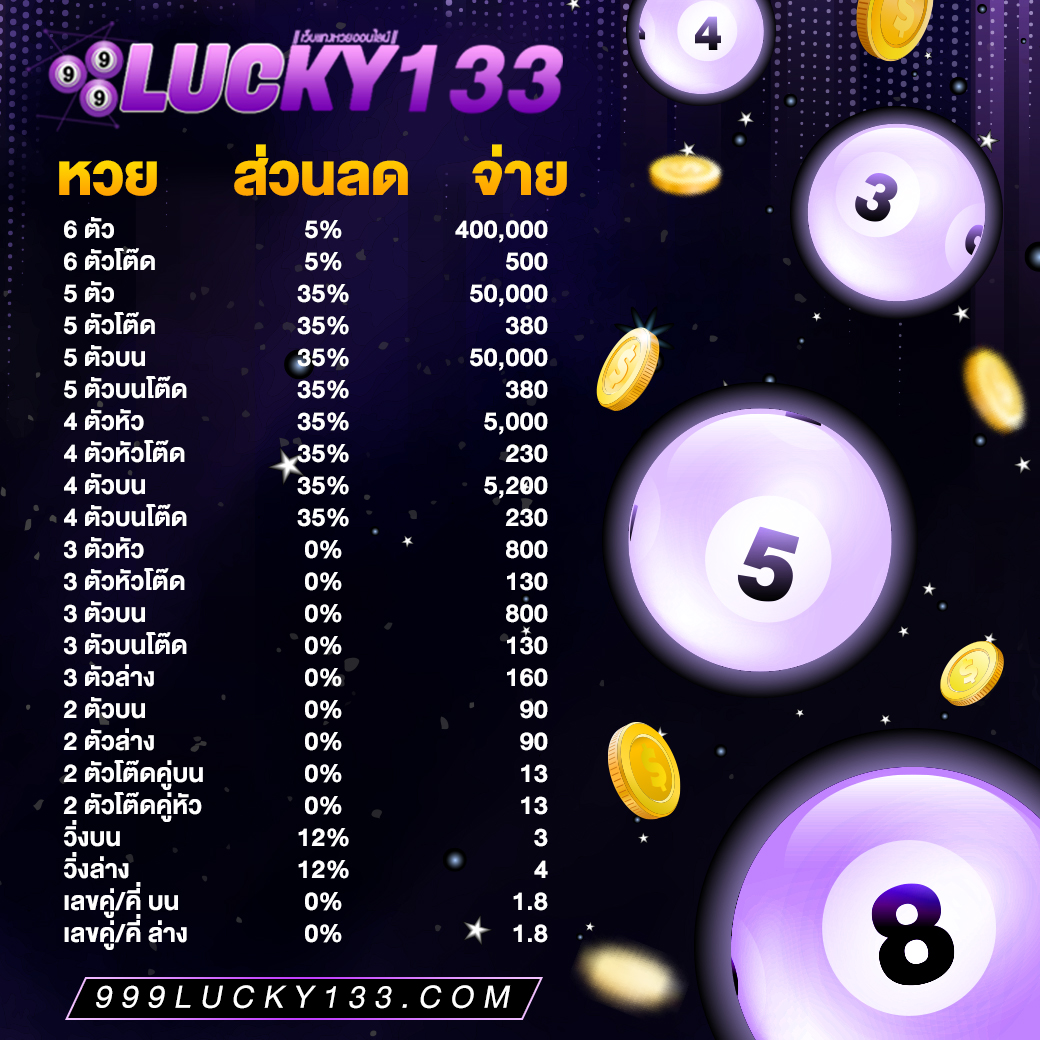
ราคาดีที่สุดในไทย หวยรัฐบาลไทย
3 ตัวบนตรง ราคาจ่ายบาทละ อัตราจ่าย 900 บาท
3 ตัวบนโต๊ด ราคาจ่ายบาทละ อัตราจ่าย 150 บาท
2 ตัวบน-ล่าง ราคาจ่ายบาทละ อัตราจ่าย
อัตราจ่าย ที่ 1
3 ตัวบนตรง ส่วนลด จ่าย บาทละ800
3 ตัวบนโต๊ด ส่วนลด จ่าย บาทละ130
2 ตัวบน-ล่าง ส่วนลด จ่าย บาทละ90
อัตราจ่าย
3 ตัวบนตรง ส่วนลด 10% จ่าย บาทละ 800
3 ตัวบนโต๊ด ส่วนลด 10% จ่าย บาทละ 220
2 ตัวบน-ล่าง ส่วนลด 25% จ่าย บาทละ 165
เว็บหวยไหนดี หวยออนไลน์
แทงหวยกับ เว็บแทงหวยออนไลน์ ไม่ว่าจะแทงเอง หรือรับมากินเปอร์เซ็นก็มีแตได้กับได้ และแผนการเล่นทั้ง 4 สเต็ปนี้ สามารถใช้ได้ทุกหวย สมัครสมาชิก ง่ายใช้เวลาไม่เกิน 5 นาที หากไม่เข้าใจหรือต้องการคำแนะนำติดต่อทีมงาน ได้ตลอด 24 ช.ม. ฝากง่าย ถอนไว ใน 2 นาที ด้วยระบบฝาก-ถอนอัตโนมัติ สมัครสมาชิก ฟรีๆ ได้เลย ด้วยขั้นตอนวิธีการ สมัคร ที่ไม่ยุ่งอยาก บนหน้าหลักของ เว็บแทงหวยออนไลน์ดันดับ1 ที่ดีที่สุดเรื่อง การเงิน
999KUCKY เว็บแทงหวยออนไลน์ ที่ไดรับความนิยมจากคอหวยทั่วประเทศ มีความน่าเชือถอนสูง มั่นคง ปลอดภัยด้วยระบบทำงานการ ฝาก-ถอน ระบบอัตโนมัติ100% อัตราจ่ายสูง แห่งปี 2020 ไม่ขั้นต่ำในการฝาก 1บาทก็เล่นได้ ถ้าหากท่านต้องการเว็บหวยดีๆ ทางเราขอแนะนำให้นักเสี่ยงโชคทุกท่านที่กำลังมองหา เว็บแทงหวยที่จ่ายตรง และสามารถใช้บริการ ฝาก – ถอน และพร้อมทำรายการผ่านเว็บที่ รวดเร็ว สะดวกสบาย ต้องเล่นกับ 999LUCKY เว็บหวยออนไลน์ เท่านั้น เรื่องเงิน หมดกังวลไปได้เลย อัตราจ่ายรางวัลสูงสุดถึง 800 บาท ซื่อตรง จ่ายจริง ไม่โกง ปลอดภัยแน่นอน มั่นใจ เรื่องเงิน เรื่องง่าย
♦ หวยของทาง 999LUCKY ♦
- หวยลาว
- หวยใต้ดิน
- หวยรัฐบาลไทย
- หวยจีบยี่กี
- หวยลาวพารวย
- หวยหุ้นไทย
- หวยหุ้นต่างประเทศ
- หวยมาเลย์
- หวยออนไลน์
- ตรวจหวย
- หวยปิปอง
- หวยหุ้นดาวโจนส์
สมัครสมชิก
เข้าสู่ระบบ
ติดต่อเรา

เปิดบริการ ฝาก-ถอน ภายใน 5 นาที
อัตราจ่ายสูง การเงิน มั่นคง ปลอดภัย ระบบอัตโนมัติ100%
